1. Introduction
Food allergies and anaphylaxis are a rising public health concern, as the prevalence and severity of reactions to common foods such as cow’s milk, peanut, tree nut, egg, fish, soybean, sesame and wheat have increased in the past few decades.1 Recent statistics reveal that 4% of children and 1% of adults have experienced food-related allergies worldwide.2 This is in comparison to Western countries, where prevalence is thought to be nearly 8% in adults and 4% in children.2 This rising trend is alarming and warrants further attention. The exact reasons for the rise in food allergens are not well understood, but many possible explanations are emerging in the scientific literature.3–5
The pathophysiological mechanisms of food allergies are complex. Food allergies are typically an immune-mediated response to proteins found in various foods, which can be either immunoglobulin E (IgE)-mediated or non-IgE-mediated.1 Symptoms can include pruritus, urticaria, nasal congestion, allergic rhinitis, gastrointestinal pain, and diarrhea.1 More severe symptoms progress to anaphylaxis, a rapidly evolving multi-system reaction that involves two or more organ systems, which can manifest as dyspnea, bronchospasm, wheezing, angioedema, and hemodynamic instability.6
Severe theories explain the increasing prevalence of food-related allergies, include the Vitamin D theory, dual allergen exposure theory, and hygiene theory.7 The Vitamin D explanation suggests that variations in serum 25-hydroxy Vitamin D (25(OH)D) levels may influence the development of food-related allergies and anaphylaxis.8 Vitamin D, which can be obtained through endogenous production, injections, supplements, and sun exposure, plays a key role in modulating immune and metabolic responses and maintaining innate and adaptive immunity.3 It has also been observed to reduce the allergic response by downregulating dendritic cells and IgE production in B-cells, as well as increasing the production of T-regulatory cells.9–11 Patients with adequate Vitamin D levels are thought to better tolerate food allergens.12 Various studies have explored factors such as sunlight exposure, geographical location, and prenatal Vitamin D levels and their role in 25(OH)D, IgE production, and immune regulation.13
Despite the lack of a universally accepted standard, sufficient serum Vitamin D are typically considered ≥ 50 nmol/L.14 According to one study, borderline insufficient levels in adults range from ≤ 50 nmol/L to ≥ 30 nmol/L, while deficient levels are considered ≤ 25 nmol/L.15 In contrast, pediatric clinical guidelines define insufficiency as 37.5-50 nmol/L, deficiency as below 37.5 nmol/L, and severe deficiency as <12.5 nmol/L.16 It is also important to note that normal Vitamin D levels can differ among racial groups due to variations in skin pigmentation, sun exposure, and dietary habits, which should be considered when evaluating Vitamin D status.17,18
The dual-allergen hypothesis suggests that exposure to allergens through damaged skin can lead to IgE production and sensitization, increasing the risk of an allergic response when the allergen is later ingested.19 Similarly, the hygiene hypothesis proposes that a lack of microbial exposure in early childhood may disrupt immune regulation, increasing susceptibility to food allergies.20 Moreover, newer hypotheses have emerged that explore genetic markers, biological factors, and the impact of the Western diet on the development of food-related allergies, though evidence remains limited.21,22
In addition to the complexity of determining the precise pathophysiology of food-mediated allergic reactions, there is a lack of agreement on when clinicians should screen for Vitamin D deficiencies. For example, the Endocrine Society recommends against routine Vitamin D testing in the absence of pre-existing health conditions.17 The lack of consensus on screening for Vitamin D deficiency may lead to discrepancies in treatment guidelines, negatively impacting medical decision-making and leading to suboptimal treatment.7,23–26
Our systematic review aims to fill a research gap by determining the association between serum 25(OH)D levels and food-related allergic responses across different age groups.
2. Methods
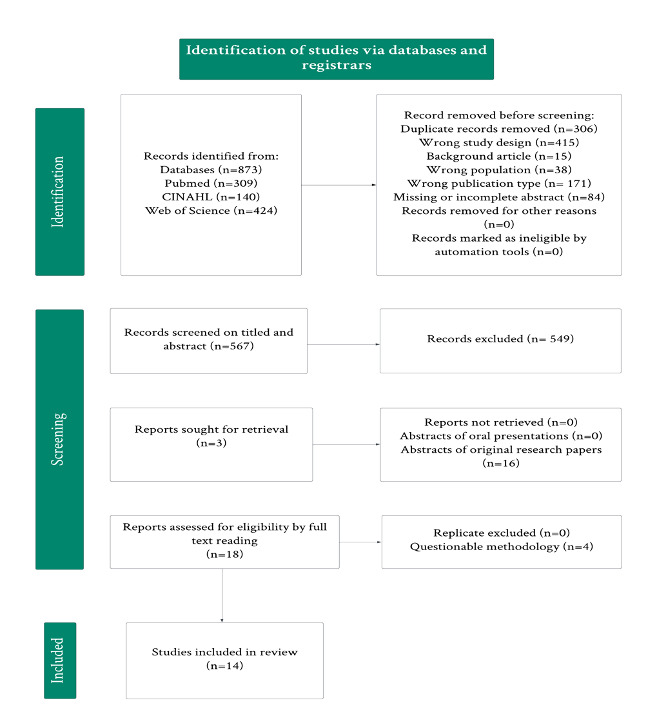
This systematic review was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) checklist and the protocol of the review was registered in-priori with PROSPERO (Record ID: 534312). PubMed, CINAHL, and Web of Science were searched using a librarian-assisted search strategy. Figure 1 shows our full strategy that we used to pool data. Following duplicate removal, two reviewers independently assessed the titles and abstracts of the search results using the Rayyan platform.
Discrepancies in article inclusion decisions were resolved through discussion, and a third reviewer was consulted. After the initial screening, full-text copies of relevant studies were obtained for further assessment. Two reviewers then independently checked the full papers for eligibility, and differences were resolved through consultation with a third reviewer.
2.1. Selection Criteria
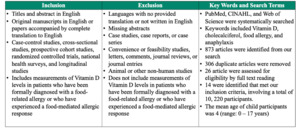
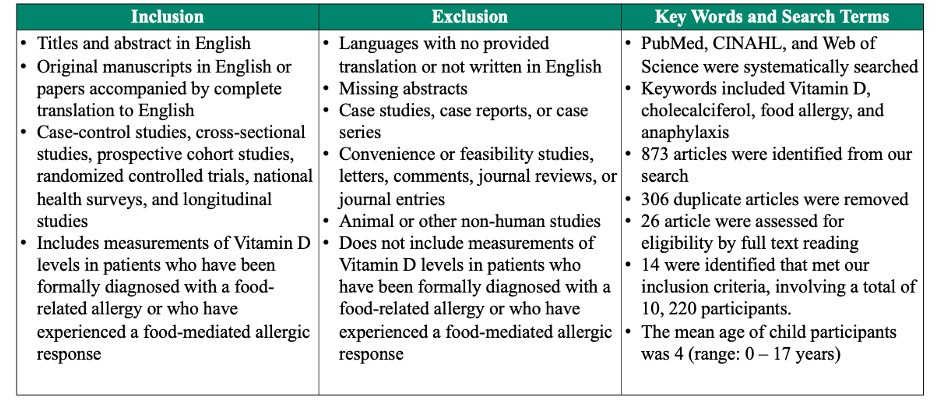
Studies were included if they analyzed the relationship between Vitamin D levels and food allergies or anaphylaxis in children and adults. We included retrospective and prospective cohort studies, randomized controlled trials, population cohort studies, national health surveys, and cross-sectional studies published in English. Studies that were not written or translated in the English language or did not include Vitamin D measurements in participants were excluded.
Additionally, we excluded literature that was not quality-appraised or peer reviewed journals. Lastly, studies were excluded if their study designs failed to include one or more of the following demographic data: age, sex, race, or ethnicity, as well as one or more of the following clinical data: medical history, family history, social history, environmental history, or food allergy history. Please see Table 1 for the full inclusion and exclusion criterion.
2.2. Data extraction
Data was extracted by two review authors and accuracy was verified by a third review author. The following study information was extracted from the included articles: title, authors, year of publication, study design, population size, and method and interval of Vitamin D assessment. Additional clinical data collected included allergy history (i.e., diagnosis, type, reaction, and severity when applicable), past medical history, past family history, past environmental history, and documented recurrence of food-related allergic reactions. Numerical data are presented in the manuscript as mean (± standard deviation when reported)
2.3. Outcome Measure
The primary outcome was serum Vitamin D levels in the food allergy group in comparison to the control group. Secondary outcome measures included regulatory T and IgE cells and their association with Vitamin D levels, as these are important markers of allergic reactions.
2.4. Data Analysis and Synthesis
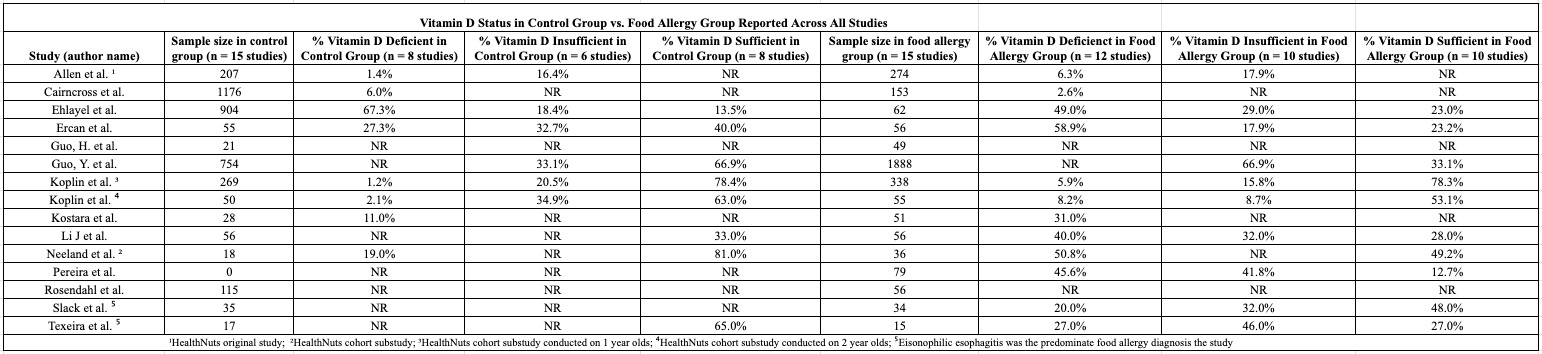
We conducted a narrative synthesis of the relationship between Vitamin D levels and food-related allergies. Table 2 provides a summary of Vitamin D deficiency, insufficiency, and sufficiency prevalence across children and adults, illustrating associations between key variables of interest. Please refer to the results section for detailed observations from this systematic review.
2.5. Quality Assessment
Two reviewers (AL, KJ) assessed study quality using a modified Oxford Centre for Evidence-Based Medicine rating scale.27 Appropriate quality assessment (QA) tools were used in accordance with the study design. Cochrane Risk of Bias (ROB 2.0) for RCTs was utilized for assessing bias.28
3. Results
Upon initial screening and removal of duplicates, 567 articles were screened with 18 articles meeting our inclusion criteria. After meticulous analysis by three authors, 14 articles met our inclusion criteria, involving 10, 220 participants. Among the 14 studies and 1 substudy included, four were case-control, four were cross-sectional, six were population-cohort, and one was a randomized controlled trial. Of note, three of our studies originated from the “HealthNuts study”, with Koplin’s article contributing two studies due to its division into cohort substudies, one focusing on infants at one year, the other on infants at two years old. This is reported as two separate studies for clarity in data reporting. These three cohort substudies encompassed a range of subjects, including infants with peanut, egg, sesame, cow’s milk allergy (CMA), and shrimp allergy, that contributed to our findings.
Key Sociodemographics and Health-Related Variables Across the Studies
The mean age of children study participants across all studies was 4 years old with a range of 0-17 years old. The mean age of adult study participants across all studies was 20 years old with an age range of 18-64 years old.
In children, the total number of males and females in the food allergy group across all studies was 628 and 557, respectively. The total number of males and females in the control group across all studies was 558 and 384, respectively. In studies that reported race (n = 4), there were a total of 50 participants in the food allergy group with Fitzpatrick Type I-II skin, 140 with Fitzpatrick III-IV skin, and 27 with Fitzpatrick Type V-VI skin. In the control group, there were 10 participants with Fitzpatrick Type I-II skin, 7 with Fitzpatrick III-IV skin, and none reported with Fitzpatrick Type V-VI skin. Additionally, there were 195 participants with “other race not specified”.
With regards to country of origin in children, the highest number of studies originated from Australian participants, with four studies comprising 703 participants in the food allergy group and 544 in the control group. This was followed by three studies from China, involving 1,993 participants in the food allergy group and 831 in the control group, and two studies from Brazil, with 95 participants in the food allergy group and 16 in the control group. Allergic rhinitis and food allergies were the most commonly reported family histories across the studies, documented in a total of 10 studies. Specifically, 283 participants in the food allergy group had a family history of allergic rhinitis, compared to 202 in the control group. Additionally, 107 participants in the food allergy group had a family history of food allergy, compared to 102 participants in the control group.
In terms of allergy types in children, egg allergy was the most frequently reported across all studies (n = 8), documented in 927 participants. Conversely, cow’s milk protein allergy was the most common food allergy, documented in 1,351 participants. Other allergies reported included beef, fruit, nuts, seafood, soybean, and wheat.
In adults, there was only one study that met our inclusion criteria. In this study, the total number of male and female study participants was 42 and 27, respectively. Male: female ratio was not provided for the food allergy group and control group. Forty-four participants in the food allergy group reported having Fitzpatrick Type I-II skin. Country of origin in this study was reported as USA for 35 participants in the food allergy group and 34 in the control group. No racial statistics, family medical history, or food allergy type were provided for this study.
Comparison of Vitamin D Levels in Food Allergy and Control Groups Across The Studies
Vitamin D levels varied in children and adults. In children with food allergies, the average Vitamin level was 26 nmol/L. In contrast, the average 25(OH)D level in control group participants (n = 3705) was 28 nmol/L. With regards to Vitamin D status, 1069 participants in the food allergy group had deficient or insufficient Vitamin D levels, whereas only 607 in the control group were 25(OH)D deficient or insufficient. Only 2035 participants in the food allergy group were sufficient, whereas 2072 in the control group were sufficient.
Regarding adult study participants, average Vitamin D levels in the food allergy group was 29 nmol/L. There was no data on the average Vitamin D levels in the control group. In terms of Vitamin D status, 36 study participants had deficient or insufficient 25(OH)D levels, whereas 33 had sufficient serum levels. 35 participants in the food allergy group were Vitamin D deficient or insufficient. No data was reported for Vitamin D sufficiency in the food allergy group nor deficiency, insufficiency, or sufficiency status in the control group. Please see Tables 2 and 3 for more information.
4. Discussion
The explosion of food allergies in the last three decades has raised the eyebrows of many in the scientific community, however contemporary research investigating the role of Vitamin D in food allergies and food-mediated anaphylaxis presents conflicting data.29,30 Our systematic review aimed to fill this research gap.
Our findings suggest an association between low serum 25(OH)D levels and food allergies across various age groups. Among the 14 articles reviewed, nine reported lower mean Vitamin D levels in the food allergy group compared to the control.31–39 This trend was particularly evident in children with egg and peanut allergies. However, this association was not found in the other five studies, which demonstrated lower or comparable Vitamin D levels in the control group.40–43 Although average Vitamin D levels in control group participants were relatively similar to the food allergy group (28 nmol/L in the former, 26 nmol/L in the latter), it should also be noted there was a difference in the sample size among the groups, as the food allergy group had significantly less participants than the control group, which may have skewed average overall Vitamin D levels.
Of the 14 studies included in this systematic review, three studies reported statistics on the correlation between 25OHD and immune markers.32,35,36 Guo et al. found that children with low 25OHD had lower circulatory Tregs, higher total IgE, and persistent eosinophilia compared to those with normal 25OHD.32 Similarly, Li observed that CMA infants with serum 25OHD lower than 50 nmol/L had significantly lower total IgE, though there was no correlation between 25OHD and circulatory Tregs.35 Lastly, Neeland reported that egg-allergic infants had higher IL-8 production and lower IL-12p70 and IL-10 production compared to non-allergic infants.36 These results are similar to previously published literature, that suggests an inverse correlation between regulatory T cells and immune-modulated responses.8 However, the existing data presents a mixed picture, and the mechanisms linking Vitamin D to Treg function remain incompletely understood.14,23
There is conflicting evidence regarding the association between Vitamin D and food-related allergies or anaphylaxis in adults, with many existing papers providing inconclusive results.24,44,45 Similarly, research on the role of Vitamin D in anaphylaxis among children remains scarce and lacks definitive data.44 However, research in this area is ongoing and evolving.7,45 According to a study published in 2017 by Marshall et al, children with deficient or suboptimal 25(OH)D levels demonstrated a higher incidence of anaphylactic reactions during rush or cluster immunotherapy.46 Nonetheless, further research is needed to establish a definitive link between Vitamin D and anaphylaxis in children.
Our systematic review distinguished itself from previous studies by examining the overarching relationship between food-related allergies and 25(OH)D across multiple studies. While other studies focused on proposed mechanisms of Vitamin D’s role in allergies, such as Murdaca et al.'s study on B cell apoptosis and autoantibody inhibition, our review adopted a broader perspective.45 Our results are consistent with previous research on 25OHD’s role in food allergies, including findings from Xin Liu et al., which linked low cord blood Vitamin D levels to an increased risk of food sensitivity in children with specific genotypes.47 Similarly, our findings align with Di et al.'s 2021 literature review, which demonstrated a nonlinear relationship between 25OHD and food allergies.30 Additionally, Li et al.'s systematic review and meta-analysis found a correlation between Vitamin D supplementation and symptom reduction in allergic rhinitis, atopic dermatitis, and asthma, particularly in cases of severe Vitamin D deficiency.48
Clinical and Community Health Implications
Our systematic review highlights important insights into the relationship between Vitamin D levels and food allergies, with implications for clinical practice and public health. Clinicians may find value in considering Vitamin D status during patient evaluations. Monitoring Vitamin D levels may become a key component in the personalized management of food allergies, particularly in high-risk pediatric groups. Understanding the patient’s Vitamin D status may help inform tailored supplementation strategies, potentially reducing the severity and sequelae of allergic reactions.
Community health providers and institutions can proactively address food allergies by incorporating Vitamin D screenings in high-risk groups, such as children with a family history of allergies or those with limited sun exposure. Early intervention through Vitamin D supplementation may attenuate the severity of allergic responses. Engaging caregivers and educating communities about the link between Vitamin and food allergies can also foster preventative measures. Public health organizations are vital collaborators with local institutions to advocate for dietary guidelines that emphasize Vitamin D-rich foods, improving public health outcomes and potentially mitigating allergy risks in vulnerable populations.
Additionally, national policy efforts could support the inclusion of Vitamin D education and supplementation initiatives with broader nutrition and allergy prevention programs. Schools, childcare centers, and community outreach programs should be equipped to provide educational materials about the importance of Vitamin D, not only in allergy prevention but overall health maintenance. By integrating Vitamin D awareness into public health campaigns and local food assistance programs, communities can take a holistic approach to reducing food allergy incidences and promoting healthier lifestyles.
Limitations
Our analysis of the association of 25(OH)D and food-related anaphylaxis revealed several limitations that may influence the reliability and generalizability of our conclusions.
First, we faced publication bias by including only full-text studies published in English, potentially omitting relevant research from short reports, conference presentations, protocols, chart reviews, case studies, and non-translated literature. Second, there is a scarcity of modern studies directly examining 25(OH)D and food allergies, particularly in adults. Recent research has focused primarily on Vitamin D’s role in conditions like atopic dermatitis and asthma, with limited data on food-related anaphylaxis.
Another significant limitation is the variability in data reporting across studies. Inconsistencies in defining 25(OH)D sufficiency, insufficiency, and deficiency, as well as varying methods of determining allergy status (e.g. skin prick versus blood tests), complicate comparisons. Moreover, demographic variability across studies – spanning different ages, health conditions, and socioeconomic backgrounds – may introduce confounding factors. Variations in sample sizes and allergens studies, along with inconsistent study design and data reporting, further challenge the comparability of results.
Our systematic review design is itself a limitation, as it identifies associations but cannot establish causation. For example, it remains unclear whether increased 25(OH)D levels are immunoprotective or simply reflect healthier immune systems.
Finally, much of the current research on our topic is relatively outdated. The latest research included in our systematic review was published in 2020.34 Many of the studies were published before 2015.31,33,36,41,43,49 Newer studies are essential to update care protocols for food allergies and anaphylaxis.
Future Directions
Future research on Vitamin D and food-related allergies should prioritize the adoption of standardized clinical guidelines for Vitamin D reporting. Additionally, there is a critical need for more randomized clinical trials, as current data remains limited. Increasing the scientific community’s focus on adults with food-related allergies and food-mediated anaphylaxis would be beneficial in advancing the field of allergy and immunology. Moreover, we advocate for a broader focus on allergens, as existing studies predominantly concentrate on Cow’s Milk Allergy and egg allergy in infants in relation to 25(OH)D deficiency. Finally, there is a pressing need for greater standardization and consistency in study methodologies. Overall, further research is essential to resolve conflicting data and better understand Vitamin D’s relationship to food allergies and anaphylaxis.
5. Conclusion
Data generally supports an inverse correlation between serum Vitamin D levels and food-related allergies in children. There is also a suggested inverse correlation between serum 25(OH)D levels and IgE levels, as well as a direct correlation between Vitamin D and Regulatory T cells in children. However, some evidence remains mixed, and the mechanisms linking Vitamin D and immune function are incompletely understood.
In conclusion, while our review highlights the potential role of Vitamin D in food allergy development, particularly in children, it also underscores the need for further research. Gaps in current understanding—especially regarding the mechanisms linking Vitamin D and immune function—must be addressed before Vitamin D screening and supplementation can be integrated into standard care protocols for allergy prevention. From a public health perspective, prioritizing uniform Vitamin D reporting and expanding research to study a broader spectrum of food allergens and age groups are critical steps toward deepening our knowledge. Additionally, ensuring methodological consistency and conducting more randomized clinical trials are essential for advancing our understanding of Vitamin D’s role in food-mediated allergies and improving overall patient and community outcomes.
Statement of Contribution
All authors made substantial contributions to the conception and design of the work, the acquisition, analysis, and interpretation of data, and drafting or revising the manuscript for important intellectual content. All authors have given final approval of the version to be published and agree to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.


_of_vitamin_d_status_among_children_and_adults_in_included_studies.png)
_of_vitamin_d_status_among_children_and_adults_in_included_studies.png)